The central dogma of molecular biology DNA-> RNA-> Protein shows the direction of flow of information of how the cells use the information stored in our DNA to make the necessary proteins. But the situation in most eukaryotes is a little more complex than that simple statement. In most eukaryotes, a gene sequence in a DNA is interrupted by non- coding information. Hence to make a protein, a cell first has to transcribe the gene (make a RNA copy of the gene, called pre-mRNA) and then modify the pre-mRNA by removing the non-coding sequence (intron) and joining the coding sequences (exons) together. The modified mRNA is then exported from the nucleus (where it was made) to the cytoplasm where the ribosome uses it as a template to make the protein. In simple English, the gene for making a proteinA looks like this "HEREabhjhdyfrhUSEndcbldfhdfmMEd ldshhglgmcFORdbfhdflhfnmc PROTEIN A". The task of the cells is to remove the gibberish and make a readable text out of the given instruction - HERE USE ME FOR PROTEINA. The cells then send this information to the ribosome (the protein factory) to make the protein.
Pre-mRNA splicing is the process in which the intronic sequences are removed within a large RNA-protein complex called spliceosome.
Why is splicing important? A spliceosme can remove the non-coding introns present in a given transcript varying combination in response to cellular cues, a process called alternative splicing. The recent completion of a draft of the human genome indicated that more than 59% of the human genes seem to be alternatively spliced (Hastings and Krainer,2001) and thus we can have more complexity (make a larger number of proteins) without increasing the number of genes present. For eg, the Dscam gene in flies has 38,000 alternatively spliced isoforms from four variable exon clusters!
More importantly, it is estimated that aberrant splicing causes about 15% of genetic diseases in humans (Philips and Cooper,2000). Thus, the spliceosome plays a critical role in generating the right template for making a protein and any abnormality in this process would be deleterious to the organism.
What do we know about this process? From genetic and biochemical experiments in the humble budding yeast, scientist have been able to understand how this process occurs. Because both the mechanism of splicing and the splicing machinery are highly conserved throughout eukaryotes, knowledge of yeast splicing gives us insights into the basic process in humans.
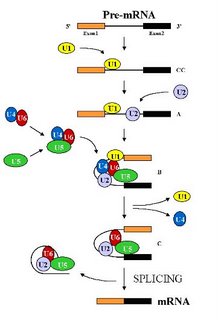 The spliceosome is the largest structure in the cell and is composed of five small nuclear RNAs ( called U1, U2, U4, U5 and U6 snRNAs) and over 100 different proteins (Stevens and Abelson , 2002). Under standard in vitro (i.e. in a test tube) assay conditions, the spliceosome assembles in a step wise manner through the addition of the U1 -> U2-> U4/U6.U5 snRNP particles (the small nuclear RNA along with its associated proteins, represented by a colored blob in the picture) on the pre-mRNA (See Figure). This assembly is an expensive process for the cell as each step consumes energy. But it also allows the apparatus to check each step and hence allows for a greater control over the overall process. Remember, a single mistake here would result in a protein that either does not function or functions abnormally. That to a cell would be hazardous and hence the cells err on the side of caution. After the assembly of the spliceosome, it undergoes structural rearrangements, resulting in the loss of U1 and U4 snRNAs, to become catalytically active (Brow D. A, 2002). Then, it proceeds to remove the intron by two transesterification reactions.
The spliceosome is the largest structure in the cell and is composed of five small nuclear RNAs ( called U1, U2, U4, U5 and U6 snRNAs) and over 100 different proteins (Stevens and Abelson , 2002). Under standard in vitro (i.e. in a test tube) assay conditions, the spliceosome assembles in a step wise manner through the addition of the U1 -> U2-> U4/U6.U5 snRNP particles (the small nuclear RNA along with its associated proteins, represented by a colored blob in the picture) on the pre-mRNA (See Figure). This assembly is an expensive process for the cell as each step consumes energy. But it also allows the apparatus to check each step and hence allows for a greater control over the overall process. Remember, a single mistake here would result in a protein that either does not function or functions abnormally. That to a cell would be hazardous and hence the cells err on the side of caution. After the assembly of the spliceosome, it undergoes structural rearrangements, resulting in the loss of U1 and U4 snRNAs, to become catalytically active (Brow D. A, 2002). Then, it proceeds to remove the intron by two transesterification reactions.
 The resultant message is released from the spliceosome along with the intron. The spliced RNA is exported to the cytoplasm for translation into the protein and the intron degraded by enzymes in the cell. The spliceosome is disassembled and the components (proteins and the snRNAs) recycled for another round of splicing.
The resultant message is released from the spliceosome along with the intron. The spliced RNA is exported to the cytoplasm for translation into the protein and the intron degraded by enzymes in the cell. The spliceosome is disassembled and the components (proteins and the snRNAs) recycled for another round of splicing.
Though much is known about the overall process, there is no insights into what triggers the activation. What informs the spliceosome that everything is set in place and hence go ahead and splice? How does the cell control the ATP driven helicases that remodel the spliceosome at each step? Or what cues the cell about abnormal spliceosome and how does it take a stalled spliceosome apart?
Next time I will try and address the role splicing plays in Humans. How does a cell choose which exon to keep? How do DNA elements present in the gene (ISEs) affect choice of exon? Does the rate at which the transcript is made affect exon choice? So keep your eyes out for Splicing -part deux.
References -
Brow D. A, Annu Rev Genet., 2002, Jun 11; 36:333-60.
Hastings and Krainer, Curr Opin Cell Biol., 2001, Jun; 13(3):302-9
Philips and Cooper, Cell Mol Life Sci., 2000, Feb;57(2):235-49
Stevens and Abelson , Methods Enzymol. 2002;351:200-20.
Check this Animation
